Boiling point. Because of this symmetrical geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane.William asked in Science & Mathematics. Chemistry · 1 decade ago. Estimate the normal boiling point of CCl4? Considering this: Delta H=32.6 kJ/mol.Colligative Properties and Determination of Molar Mass. Kb for CCl4 is 5.02 . Calculate the boiling of a 1 molal solution of naphthalene (C10 H8 ) in CCl4 .Carbon Tetrachloride (Tetrachloroethane) CCl4. Boiling point - Documents giving boiling point of elements and different kind of chemical species at varying Boiling Point of Water and Altitude - Elevation and boiling point of water.Boiling point. Because of this symmetric geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane.
Estimate the normal boiling point of CCl4? | Yahoo Answers
Hello, This table shows that boiling point of CCl4 is greater than that of SiCl4: This completely ignores the concept of greater dipole-dipole interactions in bigger molecules."CCl"_4 is completely symmetrical, and "CH"_2"Cl"_2 is not. Therefore, the latter has dipole-dipole interactions, and the former has only [However, "CH"_2"Cl"_2 does indeed have a lower boiling point than "CCl"_4. Look these up on NIST.]CCl4 has a vapor pressure of 213 torr at 40°C and 836 torr at 80°C. What is the normal boiling point CCl4?Why CCl4 has higher boiling point than SiCl4?

Kb for CCl4 is 5.02 . The boiling point of pure CCl4 is...
Carbon tetrachloride | CCl4 | CID 5943 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.An unscrupulous technician decides to dispose of 100 mL (approximately 1 mole) of CCl4 by boiling it off into the atmosphere. Estimate the percentage of C-Cl bonds in the original ~6 x 1023 molecules of CCl4 that are broken during this boiling...CCl4 is a colorless volatilize liquid at room temperature. It is slightly soluble in water. Its melting point is -22.92 ̊C (-9.256 ̊F), boiling point 76.72 ̊C CCl4 is soluble in organic solvent such as alcohol, benzene etc. CCl4 can be used as a...This preview shows page 1 - 2 out of 2 pages. normal boiling point of CCl 4 is 178°C, what is the standard molar enthalpy of vaporization of carbon tetrachloride?CCl 4 (154) 77. Notice that the boiling points of the unbranched alkanes (pentane through decane) increase rather smoothly with molecular weight, but the melting points of the even-carbon chains increase more than those of the...
Use the Clausius-Claperyon equation and resolve for ∆Hvap.
ln P1/P2 = ∆Hvap/R (1/T2 - 1T1)
P1 = 213 torr
T1 = 313 K
P2 = 836 torr
T2 = 353 Okay
R = 8.314 kJ/mol-Okay
Solve for ∆Hvap which can have devices of kJ/mole
Use the ∆Hvap and plug into the C-C equation once more, and solve for T2 the usage of P2 = 760 torr and both set of P and T from the original question.
Chemistry Archive | December 12, 2017 | Chegg.com
Answered: 1000 g solventMass % purity100[1000. g… | bartleby

Answered: Carbon tetrachloride, CCI4, has a vapor… | bartleby

PPT - Chapter 19: Chemical Thermodynamics PowerPoint ...

CH150: Chapter 7 - Solutions - Chemistry

What are some recipes for s'mores cookies?

PPT - Colligative Properties of Electrolytes PowerPoint ...

Heat of Vaporization from Vapor Pressure (Example) - YouTube

Chapter 2 solutions

Practice Chem exam 1.docx - 1 Draw 4-ethyl-trans-2-heptene ...

What Is CrO5 Butterfly Structure... - Brainly.in

PPT - Clicker Questions Chapters 13, 14, 15 PowerPoint ...

Chemistry Archive | December 04, 2016 | Chegg.com
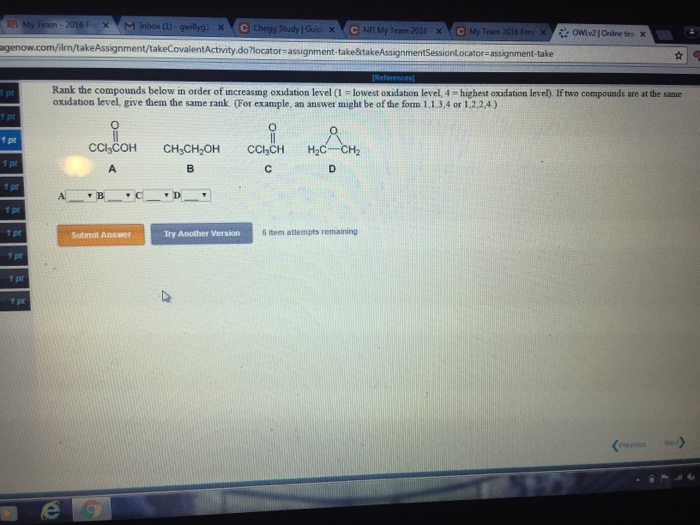
Answered: Adrenaline | bartleby

PPT - H 2 O(l) --> H 2 O(s) Normal freezing point of H 2 O ...

The equation becomes 0 G RT lnK Rearranging this becomes G ...

PPT - Clicker Questions Chapters 13, 14, 15 PowerPoint ...

PPT - Sample Exercise 19.1 Identifying Spontaneous ...
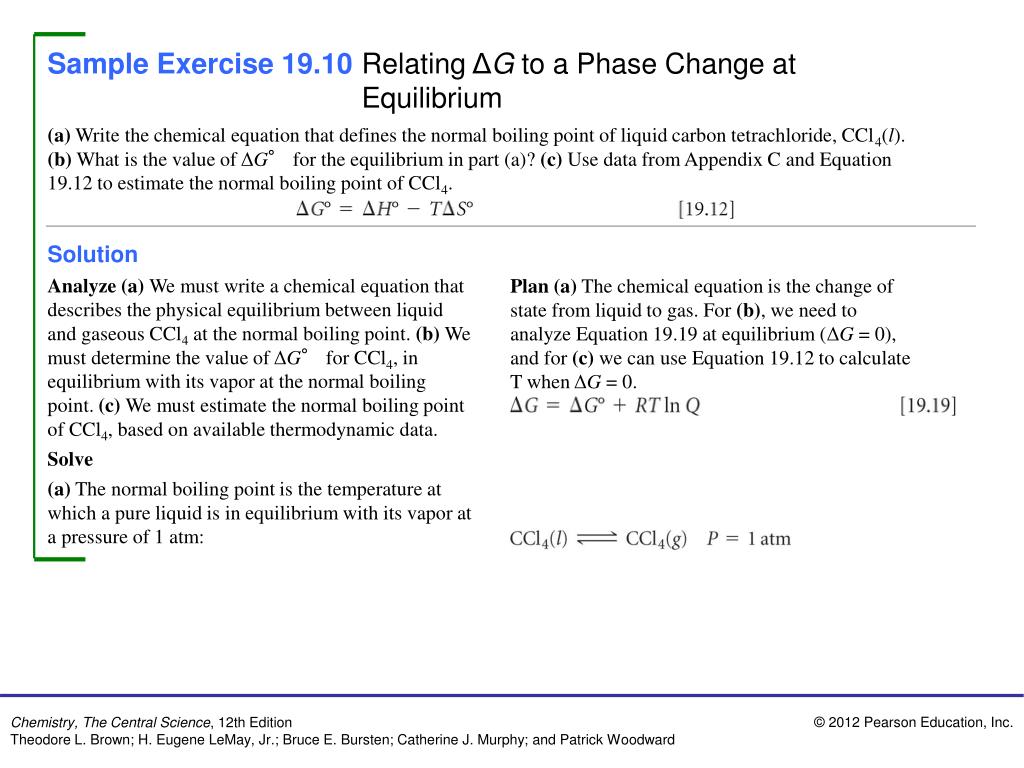
Solution

Final Exam - not including 14, 15, 16 - use other card set ...

What Is the Boiling Point of Carbon Tetrachloride?

0 comments:
Post a Comment